Describe How Changing the Particles Changed the Atom
Log in with Facebook Log in with Google. It is a story of how ideas changed about the nature of the atom.

What Are The 5 Postulates Of Dalton S Atomic Theory Atomic Theory Chemistry Lessons Study Chemistry
These responses to Parmenides suppose that there are multiple unchanging material principles which persist and merely rearrange themselves to form the changing world of appearances.
. Write the complete symbol for the atom X with the given atomic number Z and atomic mass A i Z. Close Log In. This should allow you to establish how many types of charge there are.
The forces between charged particles that are not moving or are moving relatively slowly are known as electrostatic forces. These are the notes and diagrams I use when I teach the atomic nature of matter to non-science majors. The Atomic Age also known as the Atomic Era is the period of history following the detonation of the first nuclear weapon The Gadget at the Trinity test in New Mexico on July 16 1945 during World War IIAlthough nuclear chain reactions had been hypothesized in 1933 and the first artificial self-sustaining nuclear chain reaction Chicago Pile-1 had taken place in December.
As atomic physics and chemistry began to explain the periodic table with the help of the Bohr model of the atom in the early 1900s magnetic properties were assigned to the electrons in atoms. Lave lower kinetic energy than water at 100º C. No the answer will not change upon changing the temperature and pressure because only the number of protons and mass of protons are involved.
Spiritual meaning of eyes changing color. Beginning in 1846 German physicist Wilhelm Eduard Weber theorized that electricity was composed of positively and negatively charged fluids and their interaction was governed. How many protons and neutrons are present in the following nuclei Answer.
Remember me on this computer. You can start your study by exploring the circumstances under which electrostatic forces are attractive or repulsive. In the atomist version these unchanging material principles are indivisible particles the atoms.
The atomists are often thought to have taken the idea that there is a lower limit to. The you can proceed to qualitative study of how the force between. Temperature is the term used to explain how hot or cold an object is.
Enter the email address you. Absolute zero is the temperature used to describe when all movement is as slow as it can possibly be. Electrons appeared to exhibit two types of motion in an atom.
Between 1838 and 1851 British natural philosopher Richard Laming developed the idea that an atom is composed of a core of matter surrounded by subatomic particles that had unit electric charges. Resnick - Quantum Physics Of Atoms Molecules Solids Nuclei And Particles. Water molecules at 0º C.
The best thing about this. Temperature is the average kinetic energy of particles in the substance. Orbital motion referred to the motion of an electron around the nucleus of the atom.
Connected Teaching and Learning. Connected Teaching and Learning from HMH brings together on-demand professional development students assessment data and.
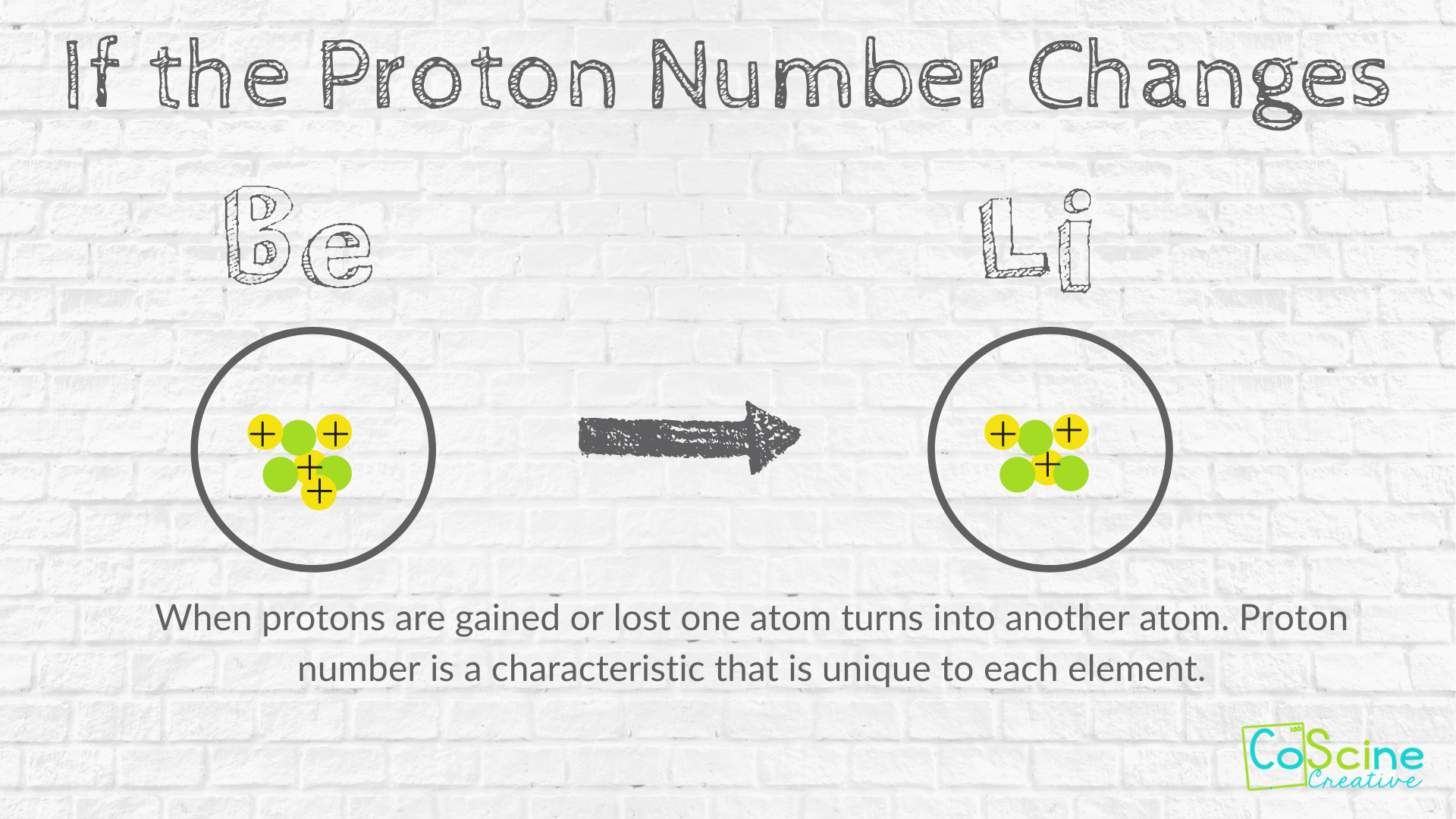
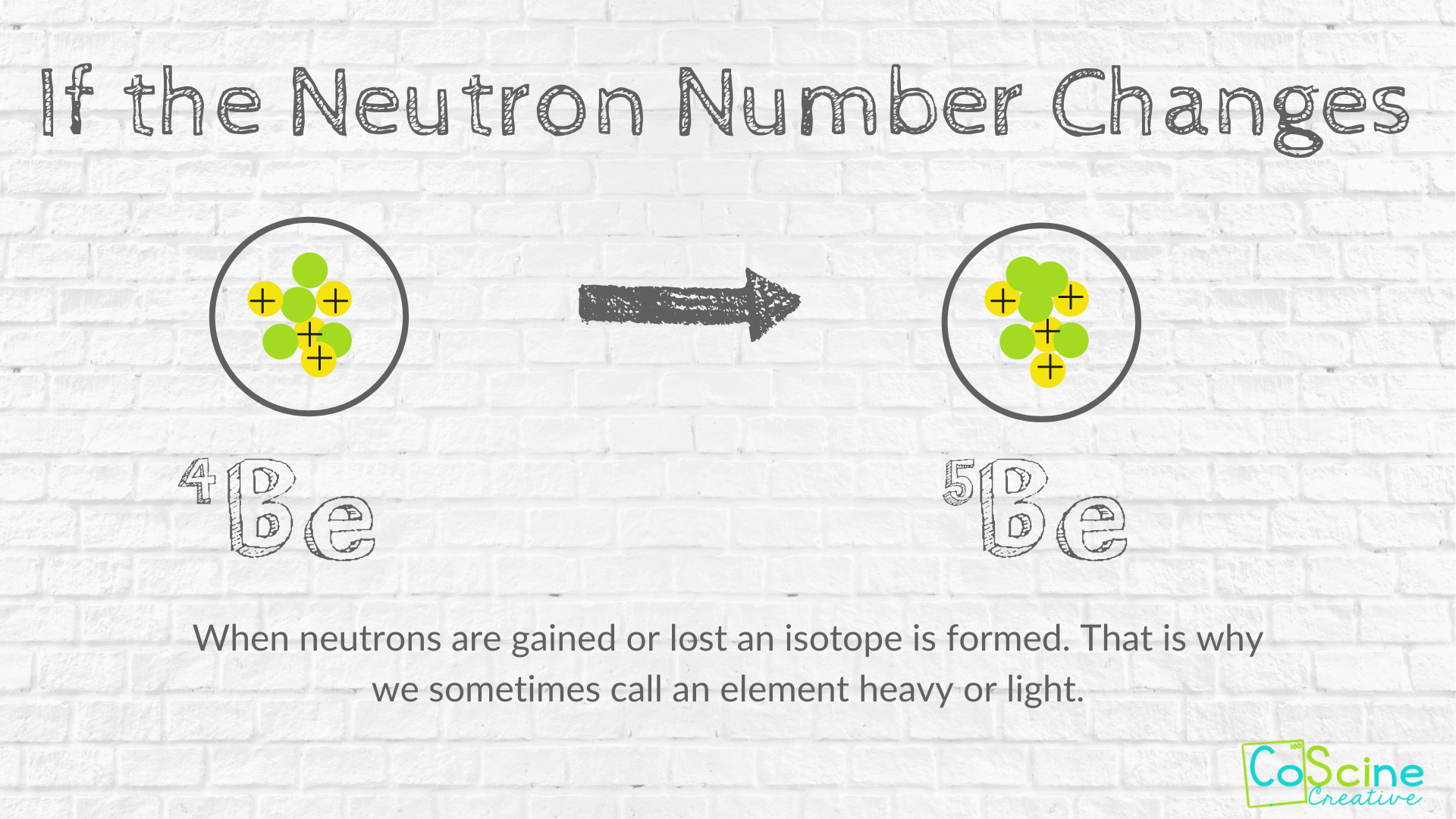
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
No comments for "Describe How Changing the Particles Changed the Atom"
Post a Comment